Peroxide Forming Chemicals (PFC) Management
Peroxide forming chemicals (PFC) are chemicals which may react with oxygen to form peroxides. This reaction is often facilitated by light. Under normal laboratory conditions this reaction may result in an increasing concentration of peroxides in a chemical container and lead to the formation of potentially explosive peroxide crystals. These crystals may explode when subjected to mechanical shock from merely opening the container.
Peroxide forming compounds fall under one of four classes:
|
Class A |
Spontaneously decompose and become explosive with exposure to air without concentration.
|
|
Class B |
Require external energy for spontaneous decomposition. Form explosive peroxides when distilled, evaporated or otherwise concentrated.
|
|
Class C |
Highly reactive and can auto-polymerize as a result of internal peroxide accumulation. The peroxides formed in these reactions are extremely shock- and heat-sensitive.
|
|
Class D |
Materials that do not fit within Group A or B or C but require special handling. |
Please see Appendix C: Peroxide Forming Chemicals Management for a list of peroxide formers in each class.
Storage
PFCs should be stored in sealed, airtight, light resistant containers and left in the original manufacturer containers whenever possible. PFCs that have been purified of inhibitors must have inhibitors reintroduced upon storage. The addition of inhibitors should be documented. Oxygen exclusion practices should be used whenever possible (i.e. purge with inert gas).
All laboratories working with PFCs must maintain an inventory in EHS Assistant to ensure these chemicals are removed from inventory before their expiration date, examined for signs of potential peroxides, and/or tested for peroxides. Laboratories should only order quantities necessary to complete an experiment and that will be used up within their shelf life. When possible, PFCs with added peroxide formation inhibitor, should be purchased.
Labelling
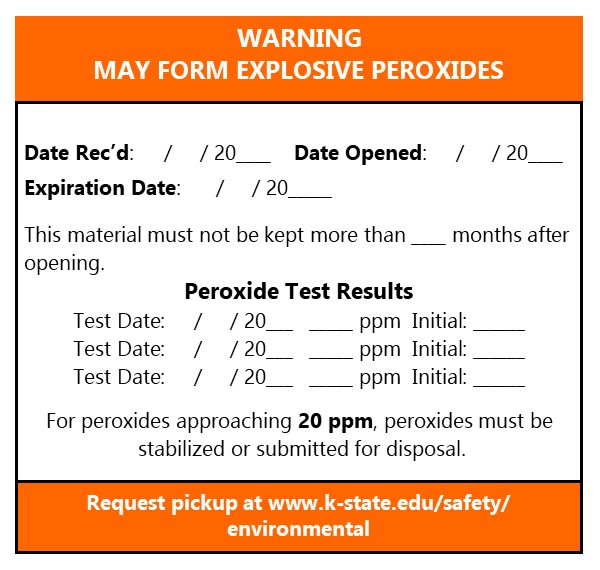
Label all peroxide forming chemicals with the EHS provided label. Document on the label:
- Date Received
- Date Opened
- Expiration Date, if available.
- Document the number of months the PFC must be disposed of after opening.
- If the PFC is tested for peroxides, record your initials, the date and concentration of peroxides in parts per million (ppm). When testing, if results continue to climb and start nearing 20 ppm, a Waste Pickup Request must be submitted before it reaches 20 ppm. If they are over 20 ppm it may require a costly hazardous waste disposal process.
Place this label on all PFC materials. It is available from EHS or may be printed by clicking the image below:
Peroxide Testing
Peroxide forming chemicals must be visually inspected for increased viscosity, crystal formation, or stratification, and age before use as these signs indicate an elevated explosion risk. It is imperative that containers exhibiting these signs not be opened as the mechanical forces could detonate the crystals. Do NOT open or test containers of unknown origin or age, or those that have evidence of peroxide formation. Contact EHS immediately if you discover a PFC exhibiting these signs.
Class A and Class C peroxide forming chemicals should be tested before use. All PFCs should be tested prior to concentration, i.e. distillation, evaporation. Do not concentrate solutions that may contain peroxides. Peroxide testing strips are available from chemical manufacturers (example: Quantofix Peroxide Test Strips [Sigma-Aldrich Part # Z249254 and Z101680]). Follow the manufacturer's instructions for using the strips/kits to ensure adequate colorimetric detection.
For peroxides concentrations of < 20 ppm, peroxides may be stabilized, or the chemicals submitted for waste disposal. Disposal companies will not transport containers with 20 ppm or more peroxide concentration. Stabilizing peroxides >20 ppm is not recommended in all cases due to the risk from the peroxides themselves and the risk from stabilization techniques.
Disposal
Contact EHS(785-532-5856) when you discover a suspect PFC container (presence of crystals, cloudiness, visible precipitate, discoloration, liquid stratification, or oily viscous layer) or unknown age. Do not open the container. Mark the container storage area or shelf with the words “Danger: Peroxide Hazard”. Instruct others not to handle the container.
It is imperative for your safety and the safety of EHS staff and waste contractors that you provide accurate information about these materials and that you responsibly manage your inventory. Submit a waste disposal request and indicate the potential hazard if applicable.
Due to the high cost of disposal and potential risks of improperly managed PFCs, departments or PIs may be charged for handling and disposal costs for PFC that are not properly dated and managed as described herein.