Research
The Aakeröy Research Laboratory
1. Significance
The physical properties of a material e.g. conductivity, density, color, solubility etc., are determined by its crystal structure, (the way in which atoms and molecules are positioned with respect to each other in a crystalline material). For example, the dramatic differences between diamond and graphite (both of which only contain carbon atoms as building blocks) are due to their crystal structures (3-D vs. planar). Today, we are still unable to predict the crystal structure of even the simplest of compounds even with complete information about the chemical composition. However, if we were able to control the structure of a solid, we would also be able to customize its physical properties. Control over the assembly of molecules into extended networks is rapidly becoming an important target in both materials chemistry and biochemistry. In this context, the selectivity and directionality of the hydrogen bond have been instrumental in the preparation of distinctive structural aggregates, and the use of hydrogen bonding as a steering force is now emerging as the most important strategy in crystal engineering
2. Objectives
2.1 Fundamental crystal engineering
(i) To examine hydrogen bonding in the solid-state and to develop strategies that employ hydrogen bonding as a tool for designing and incorporating predictable structural aggregates into organic and inorganic crystalline materials.
(ii) To explore correlations between internal architecture (structure), external appearance (morphology) and performance (properties) of crystalline materials. Special attention will be given to polymorphic and acentric systems.
2.2 Design of functional solids
(i) To synthesize new nonlinear optical material materials by ‘grafting’ polarizable chromophores onto chiral hydrogen-bonded 1-D and 2-D architectures which provide mechanical strength. These architectures are also zwitterionic leading to improved thermal stability.
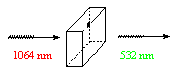
An SHG-active crystal will double the frequency of light
(ii)To construct low-dimensional solids around robust, but flexible, hydrogen-bonded sheets separated by cationic ‘pillars’. The interplanar separation is determined by the bridging cations, thus enabling the design of cavities and tunnels with specific dimensions that can be used for separations and catalysis.
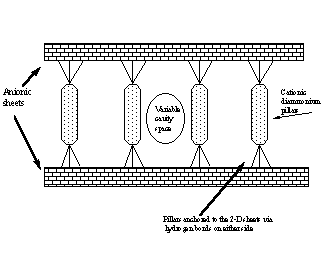
(iii) To direct the assembly and orientation of photoreactive species to enable photochemically induced dimerization reactions; the structural control will be imparted by "preformed" 1-D and 2-D anionic hydrogen-bonded motifs.
3. Previous work
3.1 Organic crystal engineering
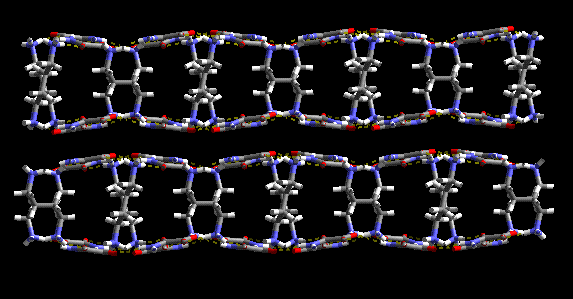
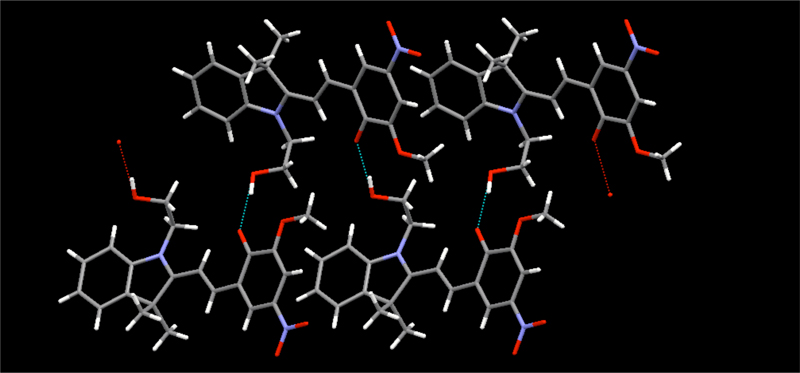
We have shown how a variety of simple molecules can be used in the predictable assembly of chains, ladders, sheets and channels.
An organic lamellar solid, constructed via hydrogen bonds
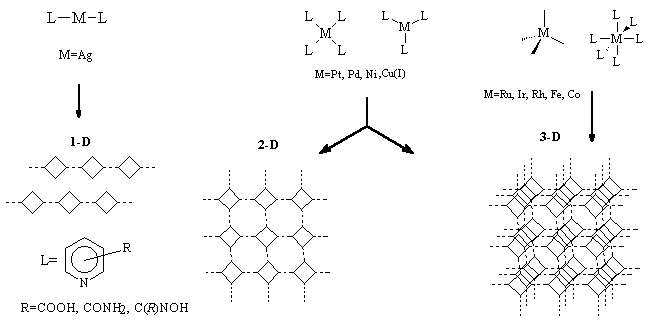
3.2 Crystal engineering and coordination chemistry
We have promoted specific coordination geometries of transition-metal complexes (linear, square-planar, octahedral) into infinite motifs with predictable architectures. These materials can have important applications in separation and catalysis.
3.3 Polymorphism
Some compounds can crystallize into different structures and below is a view of the one of the three known crystal structures of 2-amino-5-nitropyrimidine. All forms can be grown simultaneously from the same solution, and since they have different structures they also have different physical properties. Thus, a single molecule can give rise to three different materials.
Form I of 2-amino-5-nitropyrimidine
4. Synthesis of Molecular Co-crystals: A Versatile Approach to Controlling Bioavailability, Formulation, and Processing of Anti-Cancer Drugs
4.1 Introduction
Active pharmaceutical ingredients (APIs), including many anti-tumor drugs, are frequently delivered to the patient in the solid-state as part of an approved dosage type (e.g. tablets, capsules, etc.). Solids provide a convenient, compact and generally stable format to store a drug product. APIs can exist in a variety of distinct solid forms, including polymorphs, solvates, salts, co-crystals and amorphous solids, where each form may display unique physicochemical properties such as melting point, hygroscopicity, morphology, and surface activity. Each physical form will also have a profound impact on two of the most important properties that are essential to the successful development of drug candidates: solubility and stability.
4.2 Challenge of co-crystal formation
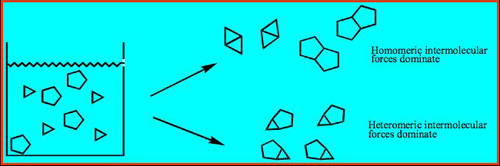
What is the most likely outcome when a homogeneous solution containing two different molecular solutes is allowed to evaporate to dryness? Unless a chemical reaction driven by the formation of covalent bonds takes place between the two solutes, one would, as a rule, expect the appearance of two separate molecular solids.This is a simple recrystallization – an event that is taken for granted as a highly effective method of purification in industrial processes as well as in every conventional synthetic laboratory. In the supramolecular laboratory, however, the very same process provides the supramolecular chemist with an opportunity to move in a completely different direction – a co-crystallization is a deliberate attempt at bringing together different molecular species within one periodic crystalline lattice without making or breaking covalent bonds.The chances of accidentally making a co-crystal and bringing together different molecular species within one periodic crystalline lattice without making or breaking covalent bonds, are very small without effective synthetic protocols, Scheme 1, so how do we go about developing reliable, effective, and versatile synthetic methods for the directed assembly of heteromeric co-crystals?
4.3 Research Program
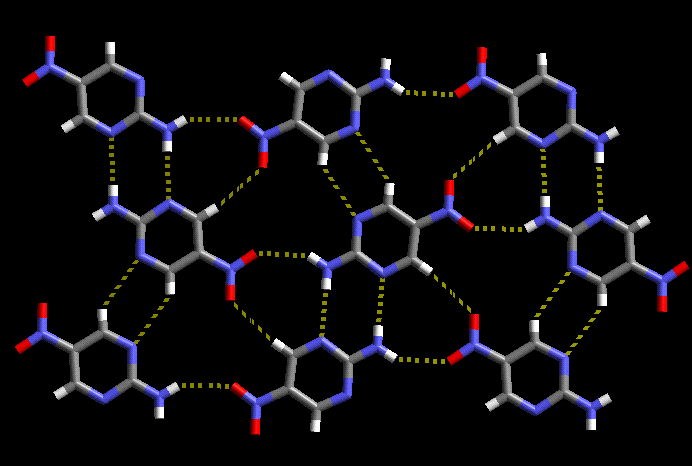
For the last five years we have carried out systematic synthetic and structural studies with the goal of developing practical, versatile and reliable strategies for the construction of molecular co-crystals. We have been able to demonstrate how specific molecular recognition events can be controlled by modulating the balance between different binding sites in a series of tailor-made supramolecular reagents (SR’s). For example, recent work on isonicotinamide resulted in the unprecedented assembly of ternary supermolecules with predictable and desired connectivity, Scheme 2.
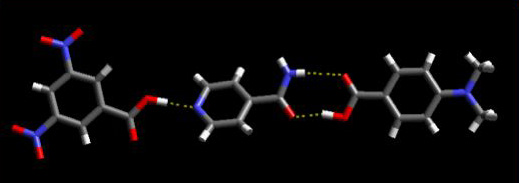
Scheme 2 A ternary supermolecule in 3,5-dinitrobenzoic acid/isonicotinamide/3-methylbenzoic acid. The stronger acid binds to the best acceptor (pyridine), and the weaker acid binds to the ‘second-best’ acceptor (amide).
We are applying our current expertise in supramolecular synthesis to the development of strategies that enable the formation of co-crystals of API’s using predesigned and readily available small molecules. After demonstrating the feasibility if this approach, we intend to initiate a major new research program geared towards the design of a library of SR’s that can be employed for making subtle changes to bulk physical properties such as solubility and stability of several classes of anti-tumor compounds without adversely affecting their inherent molecular bioactivity.
5. Supramolecular chemistry of photochromic spiropyrans: using non-covalent interactions for controlling physical properties (collaboration with Dr. Silvia Giordani, Trinity College, Dublin)
In order to understand how we can control or fine-tune the balance between open and closed forms of spiropyrans we have initiated a systematic synthetic-structural program in order to determine how (i) different substituents affect the precise molecular geometry (and thus photochemical behaviour) of the spiropyran molecule and (ii) how non-covalent interactions can be employed for shifting the intramolecular equilibrium in such a way that specific responses of the photo-active molecule can be engineered in a predictable manner. Furthermore, once we can control the availability and appearance of the open merocyanine form, Scheme 3, we are seeking to design and develop chemosensory devices geared towards selective recognition and binding of biologically/environmentally relevant molecules and metal ions.