Seek magazine
Seek is Kansas State University’s flagship research magazine and invites readers to “See” “K”-State’s research, scholarly and creative activities, and discoveries.
From the vice president for research
I am pleased to introduce the Fall 2025 issue of Kansas State University’s award-winning research magazine, Seek. Our dedication to advancing knowledge and finding solutions to global challenges shines through in the stories featured in this issue.
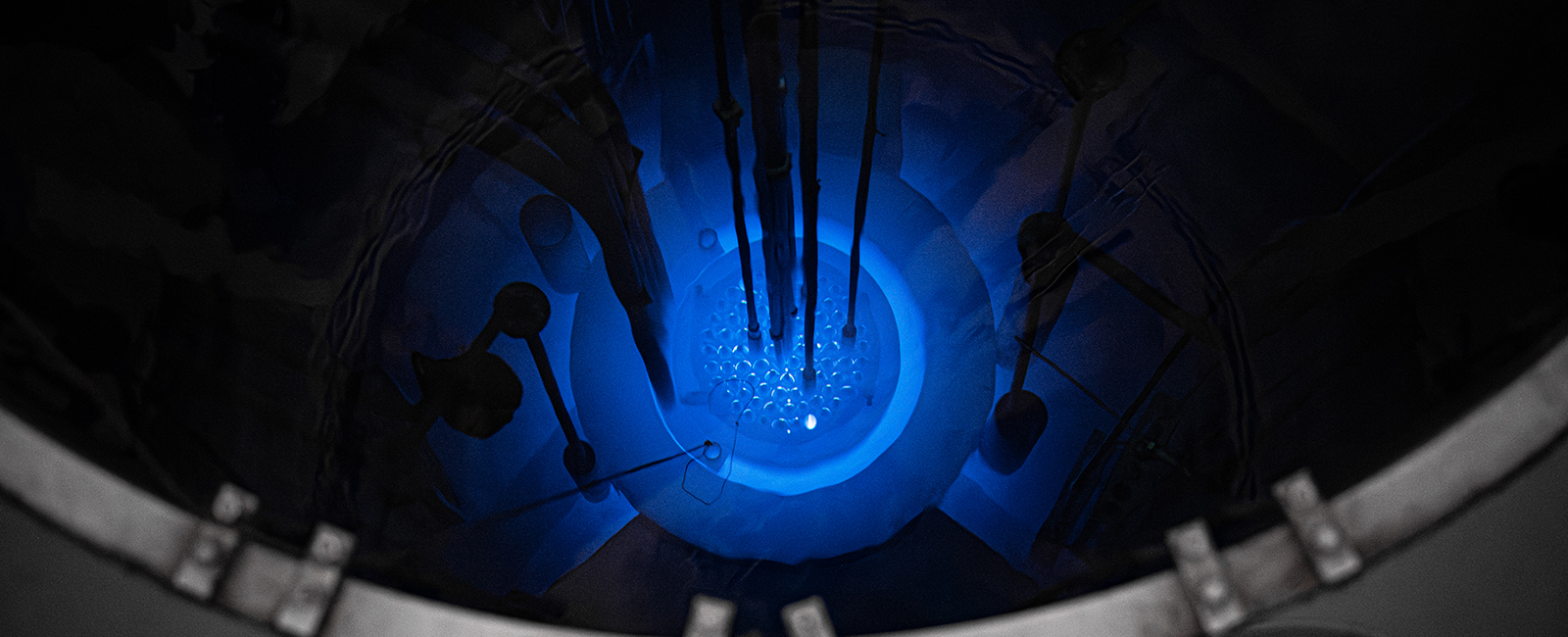
Our cover story highlights K-State’s renewed commitment to nuclear engineering. The newly resumed undergraduate program builds on more than 50 years of expertise, positioning our university to meet urgent industry needs in nuclear energy creation, radiation safety and defense. K-State’s TRIGA Mark II Nuclear Reactor continues to power essential research, from soil science to medicine, underscoring our leadership across multiple disciplines.
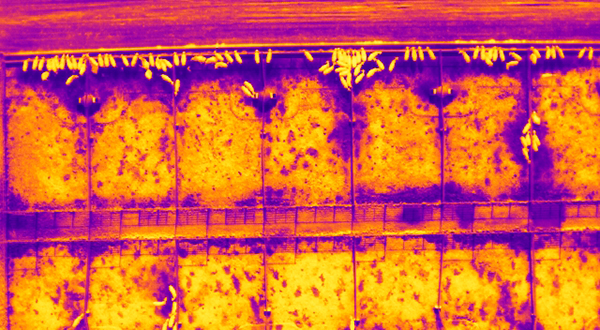
Our second feature takes readers onto the plains of Kansas, where innovative thermal imaging technology developed by Haley Larson and her team helps map cattle operations. These interdisciplinary efforts provide actionable insights for producers, allowing them to track activity and detect emissions or disease more efficiently than ever before.
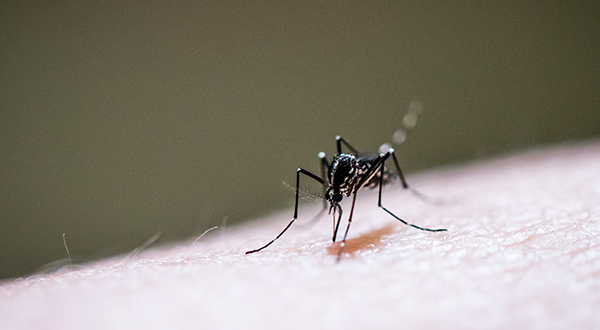
Vector-borne diseases are an escalating concern in our region, and the third feature spotlights the groundbreaking work of the Vector-Borne and Parasitic Diseases Collective. Their collaborative projects are developing new solutions to insect-related health challenges.
Staying vigilant against threats throughout the food system, our researchers direct the Great Plains Diagnostic Network, helping safeguard both Kansas agriculture and American consumers. From plant pathology to fast-evolving diagnostic techniques, K-State researchers are working to improve every stage of food production.
Also in this issue, you’ll discover how undergraduate and graduate students, like Sam Speck and Muhaiminul Islam, are tackling ecological and climate challenges through research on prairie landscapes and sustainable design. Faculty-driven innovation thrives in areas from textile dye research for green burials to multimedia advancements in music therapy, reflecting the creativity and impact of K-State scholars.
As K-State builds its on its mission to be a force of good for Kansas and for the world, we’re developing cutting-edge solutions and innovations that develop industries, attract new businesses and engage communities with the expertise and resources of a next-generation land-grant institution.
These stories showcase the diverse, interdisciplinary spirit of research at Kansas State University. I invite you to explore the passion, innovation and commitment of our faculty, staff and students — and to join us in shaping the future through discovery.
Hans Coetzee, Ph.D.
Vice President for Research
Sky-high sustainability
Graduate landscape architect bolsters green roof resilience
The color collector
Fashion professor weaves sustainability into natural dyes
Conversation through concert
University Distinguished Professor's musical performances transcend words and nations
Little mouse on the prairie
Undergraduate finds big ecological role from tiny creature