CRISPR connections: How scientists use genetic technology to improve our world
By Jennifer Tidball
The solution to some of the world’s most devastating animal and plant diseases often can be found in a single gene on a DNA strand.
Finding these solutions used to take scientists years or even decades. The work is even more difficult with incredibly complex genomes like wheat, which includes 16 billion base pairs in its DNA code. To compare, the size of the human genome is only 3 billion DNA pairs.
But thanks to a revolutionary new gene-editing technology called CRISPR-Cas9, researchers have an easier and more efficient way to work with complex genomes.
Kansas State University researchers know the value of CRISPR, and they’re developing ethical and responsible ways to use this technology to improve our world, from developing new wheat varieties to fighting animal diseases.
“This new technology uses the natural processes in cells to edit or hybridize a gene to select for a specific trait in the cell as it reproduces,” said Peter Dorhout, K-State’s vice president for research who has presented to congressional staff and other national organizations on the importance of CRISPR technology.
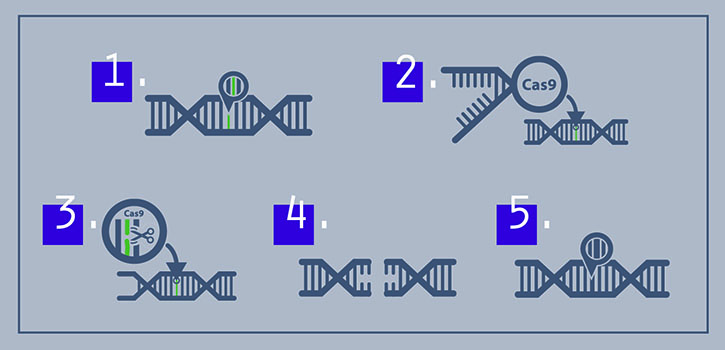
CRISPR — pronounced “crisper” — stands for Clustered Regularly Interspaced Short Palindromic Repeats. CRISPR-Cas9 is a simple, but powerful, genome-editing technology that targets and cuts DNA with an enzyme called CAS9. A genome comprises the complete set of DNA genes bundled together as the chromosomes in cells.
Since it was introduced in 2012, CRISPR technology has revolutionized the scientific world. Using CRISPR, researchers like plant pathologist Eduard Akhunov can start developing wheat breeds that have higher yield or are more drought tolerant. Biochemist Gregory Finnigan can study model species like yeast. Virologist Raymond “Bob” Rowland can combat devastating swine diseases.
The process starts with a genetic blueprint: DNA. The CRISPR-Cas9 enzyme works like molecular scissors that make cuts at specific places in DNA. Then scientists can either let the cells fix the DNA through an error-prone repair process that knocks out specific genes or scientists can supply cells with a short template of new DNA to repair the cut and simultaneously introduce a precise change to the gene.
It may seem synthetic, but a species’ DNA constantly changes because of the ongoing natural mutation process.
“All breeding, whether in crops or animals, depends on these naturally introduced mutations,” Akhunov said. “CRISPR-Cas9 can do the same thing as the natural mutation process, but it is doing it at a very fast pace and only in a specific gene in the genome.”
“Hybridizing plants, for example, has existed for millennia to utilize a plant’s own reproductive systems to create a new plant with hopefully the best traits of the parents,” Dorhout said. “However, this process can take many years and many trials to breed the best traits possible. CRISPR technology enables scientists to do this much more efficiently and effectively.”
CRISPR is a part of the immune system that protects bacteria from viruses. In fact, CRISPR was used to develop virus-resistant bacterial cultures for dairy production, which means if you ate a yogurt parfait for breakfast, you likely ate bacteria carrying CRISPR.
Researchers can use CRISPR-Cas9 technology to further explore many plant and animal genomes. The sequencing of so many genomes in the past few decades is exciting, Finnigan said, but the capabilities of CRISPR technology are even more exciting because they give scientists like him a new tool kit.
“With this new technology, we can ask questions that are bigger, more complicated and closer to a disease,” Finnigan said. “Instead of studying one or two genes, we can study hundreds of genes. Now we can do really exciting science.”
Editing genes

Eduard Akhunov, professor of plant pathology, works on research that uses CRISPR technology to develop wheat varieties.
As a genetic code, DNA defines all desirable traits in wheat, including higher yield, disease resistance, drought tolerance and end-use quality.
In the College of Agriculture, Eduard Akhunov, professor of plant pathology, and Wei Wang, postdoctoral researcher in plant pathology, are using CRISPR technology to develop wheat varieties with improved traits. The Kansas Wheat Commission is supporting their efforts.
“We are looking at traits that can benefit the Kansas farmer and wheat production in Kansas,” Akhunov said. “Then, we are trying to edit genes affecting these traits and transferring them into Kansas wheat varieties.”
Thanks to the recently sequenced wheat genome, Akhunov’s team can use computational tools to find specific pieces of the wheat genome that they would like to fix. Because CRISPR is so precise, the scientists can use it to fix a few DNA base pairs to improve the wheat.
The team already has seen success: They have used CRISPR to replace a few DNA base pairs and create wheat lines with increased grain weight and improved protein content.
To create a new wheat variety using traditional breeding methods can take at least five to seven years because scientists must crossbreed different varieties, or sometimes even distantly related species, to introduce desirable traits. Using CRISPR technology, Akhunov and his team can introduce novel traits into wheat varieties in about two years by editing specific genes that control these traits.
CRISPR technology especially can be useful in cases when new devastating wheat diseases emerge quickly and rapid development of resistant varieties is critical for maintaining crop production.
“Currently, for example, we are looking for genes that can make wheat resistant to multiple fungal pathogens,” Akhunov said.
Genetic and molecular biology studies in cereal crops — such as rice, barley and corn — have identified a number of genes that control major traits across species.
“CRISPR-based gene editing has emerged as a powerful technology that can take full advantage of the available genomic information and help to rapidly transfer traits from other crops into wheat cultivars,” Akhunov said.
Akhunov and his team are applying CRISPR technology to improve a wide range of traits that includes yield, resistance to pathogens, end-use quality and nutritional quality.
Driving genes

Gregory Finnigan, assistant professor of biochemistry and molecular biophysics, and his team of graduate and undergraduate students are studying gene drives through the model system of yeast.
If CRISPR is the “car” of genetic technology, then a biochemistry team is studying the car’s engine and GPS unit.
In other words, Gregory Finnigan, assistant professor of biochemistry and molecular biophysics in the College of Arts and Sciences, is improving a piece of CRISPR technology: gene drives.
“We’re looking at the design, the architecture, how the gene drive works and how it can work better,” Finnigan said. “We want to make sure the gene drive ‘drives’ with an off switch.”
Through gene drives, genetic changes made by CRISPR can pass to offspring and future generations and can be used to direct the traits of biological populations. Finnigan and his team of graduate and undergraduate students are studying gene drives through the model system of yeast. Specifically, they are studying baker’s yeast, which is used to make bread and ferment wine.
“Our interest is studying the CRISPR technique itself, and we’re looking at including additional control to this unique system,” Finnigan said. “There’s a way to do this so that everybody wins. You can combat diseases, but you can also still preserve and protect the natural environment.”
Finnigan’s laboratory is one of a handful of laboratories worldwide that specializes in gene drive technology. By working in a single-celled organism like yeast, Finnigan can study gene drives quickly and efficiently. His work has wide applications and can benefit agriculture, humans, ecology and research itself.
For instance, Finnigan’s team has developed a gene drive with a genetic “switch” to turn off Cas9 when it is no longer needed. The team’s goal is to develop such safety mechanisms so that gene drives can be used ethically in the future.
“We want to study how to more effectively control gene drives and build safer systems,” Finnigan said.
Breeding genes

Raymond “Bob” Rowland, professor of diagnostic medicine and pathobiology, has several collaborative projects that use CRISPR to address some of the headline-making swine diseases, such as African swine fever and porcine epidemic diarrhea virus, or PEDv.
CRISPR technology is helping veterinarians and researchers fight animal diseases with a new tool: genetics.
“Whether you are talking about vaccines, diagnostics or breeding animals that are more resistant to diseases, it’s all about genetics,” said Raymond “Bob” Rowland, professor of diagnostic medicine and pathobiology in the College of Veterinary Medicine, who studies swine diseases. “CRISPR technology is changing things.”
CRISPR can cut down research time from years to months, which is important in a world where swine diseases can develop and devastate quickly. Rowland has several collaborative projects that use CRISPR to address some of the headline-making swine diseases, such as African swine fever and porcine epidemic diarrhea virus, or PEDv.
Rowland also has used CRISPR to address the most devastating disease in the swine industry: PRRS, or porcine reproductive and respiratory syndrome. The disease costs the U.S. pork industry more than $600 million in losses every year, according to researchers.
Through a collaboration with Randall Prather, a professor at the University of Missouri, Rowland and a team have developed PRRS-resistant pigs. Using CRISPR-Cas9 technology, the researchers have found that pigs without the CD163 protein show no signs or evidence of being infected with the PRRS virus. CD163 is the receptor for the virus.
Rowland also has found that mothers without the CD163 protein are resistant to the PRRS virus and give birth to healthy, normal piglets. The research can save swine producers millions of dollars because pigs are protected from the PRRS virus during the critical reproductive process, Rowland said. The pigs without the CD163 protein are undergoing further evaluation before they become available to producers.
Rowland has added swine coronaviruses to his area of study and CRISPR is helping him develop ways to make swine resistant to multiple pathogens and viruses at once.
Additionally, pigs are a good model species for studying disease in humans and other animals. The ways that Rowland and his collaborators treat diseases in pigs also could help cure diseases in humans, such as multiple sclerosis or diabetes.
“Our job as academics is to use the latest technology so that the world becomes different and better because of something we have done,” Rowland said.