Research Overview
Research Program I: Organofluorine Chemistry
New Reactions and Methodology: Fluorination and Fluoroalkylation
Installation of fluorine or fluoroalkyl (RF) groups into organic molecules imparts exceedingly important and unique biological properties. Accordingly, organofluorine compounds are known to exhibit superior metabolic stability, binding affinity, membrane permeability, and enhanced pharmacokinetic profiles in some instances. Consequently, more than 20% of newly approved pharmaceuticals and approximately 40% of registered agrochemicals possess one or more fluorine atoms.

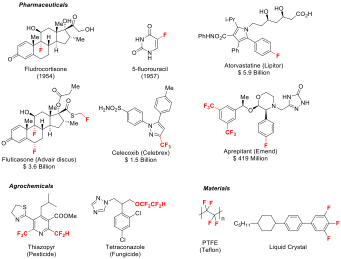
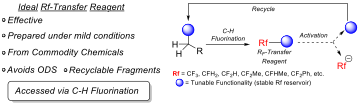
Our group focuses on developing new, efficient, and selective ways to forge C–F bonds, as well as designing new methods and reagents to perform effective C–RF bond formation, (RF = CF3, CF2H, CH2F, CF2Me, etc.) Our reagent design is guided and inspired by sustainability principles, thus it always considers the fluorine atom supply chain . Accordingly, we strive to design recyclable fluoroalkylation (RF-transfer) reagents avoiding Ozone-Depleting Substances (ODS) in their preparation.
Research Program II
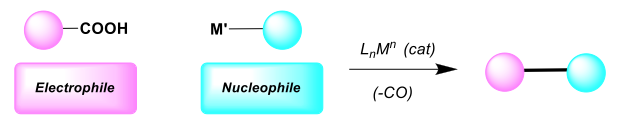
Expanding the Frontiers of Utilization of Carboxylic Acids as Electrophilic Coupling Partners in Cross-Coupling Reactions.
Carboxylic acids (RCOOH) and their derivatives (acyl halides, anhydrides, esters, amides) are among the most common functionalities encountered in organic chemistry. Due to the multiple reaction pathways available, a large number of different product classes have become accessible from this type of functionalities. Classical uses involve the utilization of their activated derivatives (acyl halides, anhydrides or esters) in acylation chemistry. However, transition-metal catalysis can enable distinct reactivities (i.e. acylative cross-coupling of acyl electrophiles). The direct use of RCOOH as substrates in catalysis is highly desirable because of their vast structural variety, commercial availability, synthetic availability (great number of preparative methods), physicochemical properties related to storage and handling, and their vast abundance in naturally occurring compounds.
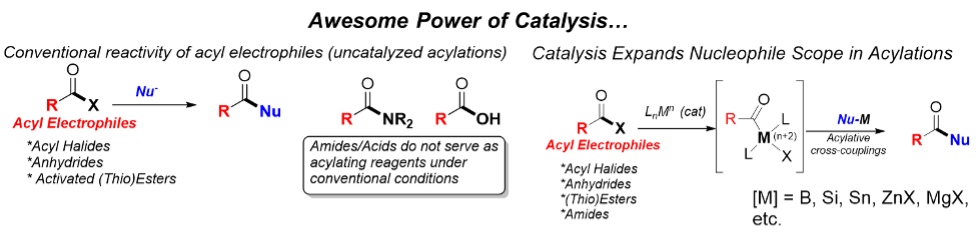
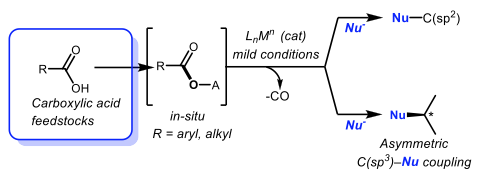
In addition to the direct utilization of carboxylic acids in acylative cross-coupling reactions, our group focuses on the development of catalytic transformations proceeding via decarbonylation, thereby effectively and directly using carboxylic acids as electrophilic coupling partners in cross-coupling reactions via CO release.
Decarbonylation Transformations are Enabled by Catalysis
Direct use of Carboxylic Acid Feedstocks in Catalytic Transformations
Research Program III
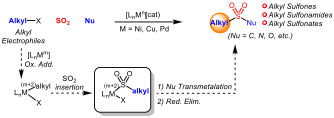
Expanding the Frontiers of SO2 Utilization in Catalytic Synthetic Transformations
Sulfonyl-containing compounds, represent an important class of substances that have found widespread application in many areas varying from materials science and agrochemical development, to medicinal chemistry and organic chemistry where they function as important building blocks or as highly specialized reagents for synthetic applications. The most prevalent compounds that contain the SO2-motif are sulfones, sulfonamides, sultams, sulfonic acids, sulfonates, sultones and sulfonyl halides, among others. Sulfur dioxide (SO2) has been recognized as major environmental pollutant that has several adverse effects on the human health. Thus, catalytic transformations of this environmental pollutant into value-added compounds is a highly desirable goal.
Compared to carbon monoxide, the organometallic chemistry of SO2 is still underdeveloped
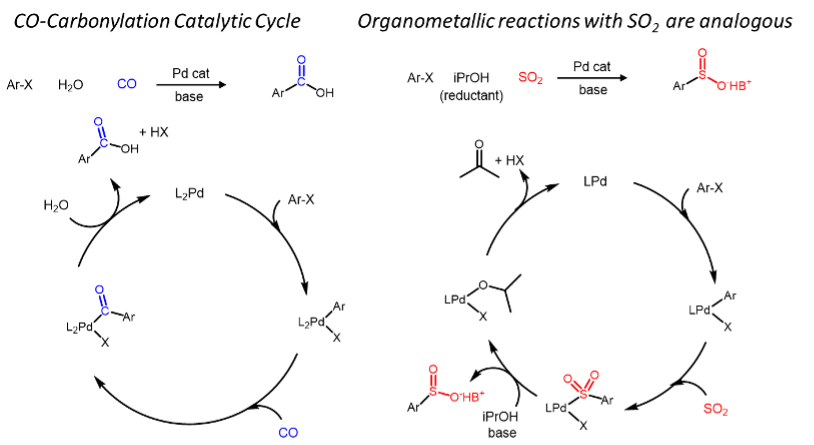
One of our main goals withing this chemistry is to develop novel sulfonylative cross-couplings of alkyl electrophiles.