Healthy animals, healthy humans: Zoonotic disease research targets maladies that infect both animals and people
By Sarah Caldwell Hancock
Diseases that spread from animals to people sicken tens of thousands of Americans each year. Some of these diseases are familiar, such as the flu, and others are largely unknown in the U.S., such as Rift Valley fever. Some are transmitted by direct contact with animals, but others are passed along by mosquitoes or ticks.
All of them are described by the same adjective: zoonotic.
Protecting humans from zoonotic diseases requires understanding the complex interactions between animal and human health. One concern is that diseases could spread around the world if the wrong person or animal travels at the wrong time.
Kansas State University researchers are fighting many of the nation’s and the world’s most devastating zoonotic diseases.
“K-State research is crucial to national security and public health,” said Peter Dorhout, K-State vice president for research. “We study several diseases that are priorities for the National Bio and Agro-defense Facility, and as we do this work, we are training the workforce needed to provide future biodefense.”
Rift Valley Fever
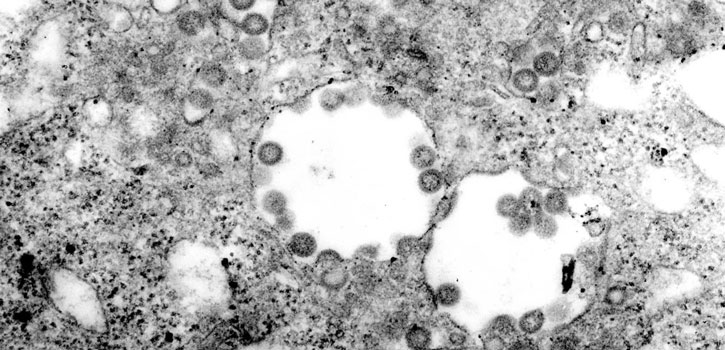
Rift Valley fever virus can cause fatal illness in humans, who contract the disease by handling infected animals or animal products. (Photo credit: CDC/F. A. Murphy; J. Dalrymple)
Rift Valley fever virus, transmitted by mosquitoes, causes abortions in cattle, sheep and goats and can kill young animals. The virus also causes severe fever in infected animals and can cause fatal illness in humans, who contract the disease by handling infected animals or animal products.
Although Rift Valley fever has not reached the U.S., it has devastated other areas of the world. According to the World Health Organization, an outbreak earlier this year killed more than 950 animals in Kenya, Uganda and Rwanda from June 22 through July 2.
Human deaths reached the double digits. In 2006, the virus killed 150 people in Kenya.
Ongoing U.S. Department of Homeland Security- sponsored research and training at the K-State Biosecurity Research Institute is helping develop and improve vaccines. A team from the K-State Center of Excellence for Emerging and Zoonotic Animal Diseases, or CEEZAD, collaborated with U.S. Department of Agriculture Agricultural Research Service scientists to develop and patent a safe subunit vaccine. The vaccine uses only a specific protein from the virus rather than the whole particle, and the team has licensed it to a private company. The group also confirmed that the common native white-tailed deer is susceptible to infection by the Rift Valley fever virus. Read more about research on white-tailed deer.
“Our work is an example of how collaborative and translational research can result in a tool to control this devastating disease if it ever comes to our shores,” said Jürgen Richt, director of CEEZAD and Regents distinguished professor of veterinary medicine.
Japanese encephalitis
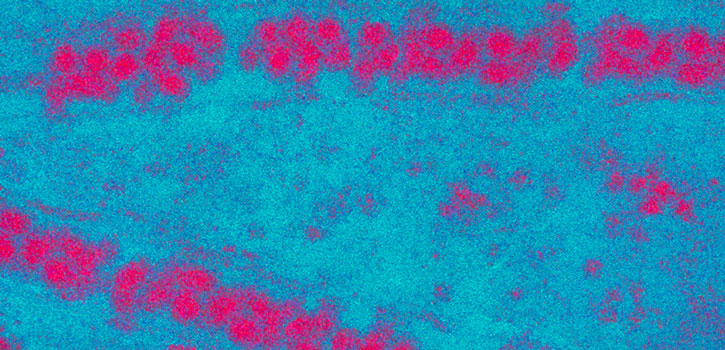
Japanese encephalitis virus thrives in pigs and wading birds and is transmitted to humans by infected mosquitoes. (Photo credit: Sanofi Pasteur/Alain Grillet)
Japanese encephalitis virus is a relative of West Nile virus and is the leading cause of vaccine- preventable brain inflammation in Asia and the western Pacific, according to the Centers for Disease Control and Prevention. The virus thrives in pigs and wading birds and is transmitted to humans by infected mosquitoes. Although most infected people do not develop symptoms, a small percentage experiences sudden onset of headache, high fever and other dangerous symptoms. Around 1 in 4 cases is fatal and a total of about 13,000 to 20,000 people die each year.
College of Veterinary Medicine researchers Dana Vanlandingham, associate professor of diagnostic medicine and pathobiology, and So Lee Park, third-year veterinary medicine student and concurrent doctoral student in pathobiology, recently co-authored a study demonstrating that North American domestic pigs could be susceptible to Japanese encephalitis virus. That means if the virus is introduced in the U.S., it could take hold and ultimately infect both pigs and humans. This research was supported by the U.S. Department of Agriculture Agricultural Research Service and its scientists in Manhattan.
Studying foreign animal diseases and understanding their transmission cycles is an important step toward preparedness, Vanlandingham said. The U.S. learned this lesson the hard way with West Nile virus. Since 1999, West Nile virus has infected five million people and killed several thousand people.
“This sort of information would have been useful for past introductions such as West Nile virus, which is similar to Japanese encephalitis virus,” Vanlandingham said. “Had we studied West Nile virus prior to its arrival in the U.S., we may have been better able to minimize the spread when it came into New York in 1999.”
Influenza
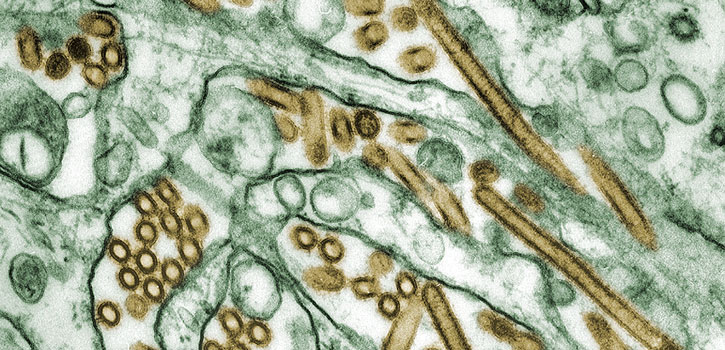
Influenza virus strains can originate in avian, swine or bat populations and spread to humans. (Photo credit: CDC/Cynthia Goldsmith; Jacqueline Katz; Sherif R. Zaki)
Coughing co-workers mumble about having “the flu,” but they may not know that their illness may have originated in animals. After the flu, or influenza, makes the initial jump from animals to humans, humans spread it quickly through coughs and sneezes.
Wenjun Ma, associate professor of diagnostic medicine and pathobiology in the College of Veterinary Medicine, studies different strains of influenza, including highly pathogenic avian influenza and other strains that can afflict poultry as well as swine influenza and bat influenza. A previous collaboration with Jürgen Richt, Regents distinguished professor of veterinary medicine, and Mount Sinai Health System researchers developed a vaccine that easily could be delivered to entire poultry flocks to protect them from multiple avian influenza strains.
The overall goals of Ma’s research are to understand why influenza can cross species barriers and how the viruses are transmitted. Bat influenza A-like viruses are one of Ma’s targets. These recently discovered viruses cause concern because humans and other species have no immunity against them.
“If a novel virus is able to infect humans and transmit human to human, it can cause a pandemic with higher fatality,” Ma said. “Almost nothing is known about these viruses. That’s why our research is so important.”
Zika
Zika virus was first identified in monkeys and is spread among humans by certain mosquito species. (Photo credit: CDC/Cynthia Goldsmith)
Zika virus was all over the news just two summers ago, but scientists remain uncertain about why infections have declined sharply. This virus is spread by certain mosquito species and causes potentially fatal birth defects in babies whose mothers were infected during pregnancy. Last year, 437 people in the U.S. were infected with Zika while traveling abroad, according to the Centers for Disease Control and Prevention.
A resurgence is possible, and K-State researchers are helping the world prepare.
Stephen Higgs, director of K-State’s Biosecurity Research Institute, and Dana Vanlandingham, associate professor of diagnostic medicine and pathobiology in the College of Veterinary Medicine, co-edited a book released earlier this year, “Chikungunya and Zika Viruses: Global Emerging Health Threats.” The book provides both historical and current information on these important viruses.
“Zika is an example of how quickly something can emerge,” Higgs said. “It was an obscure virus that came from Africa and spread rapidly and widely to more than one million people in a short time. We were completely unprepared for it.”
Higgs said K-State and Uniformed Services University researchers collaborated with the National Institutes of Health to evaluate new vaccines against Zika.
The team, along with Yan-Jan S. Huang, K-State research assistant professor of arbovirology in the College of Veterinary Medicine, also co-authored a paper that discovered a unique antibody effect between Zika and dengue viruses. So-called cross- reactivity may mean that as vaccines for Zika and dengue are developed and approved, people need to receive them at the same time to avoid the antibodies from one virus enhancing the other, researchers said.
Shiga toxin-producing E. coli
Shiga toxin-producing E. coli can infect humans when they eat contaminated food, such as contaminated beef or veal. (Photo credit: CDC/Janice Haney Carr)
Contaminated food is a major source of zoonotic disease spread. Most consumers have heard of Escherichia coli, or E. coli, and know that it’s something to be avoided, but only a few strains sicken people. The types that produce Shiga toxin, known as STEC, can cause illness with symptoms that include stomach cramps, diarrhea, vomiting and fever. Some infections can be life-threatening. STEC strains cause an estimated 265,000 illnesses in the U.S. each year according to the Centers for Disease Control and Prevention, with 36 percent of illnesses attributed to the worst STEC strain type: O157.
Randy Phebus, professor of animal sciences and industry in the College of Agriculture and interim director of the Food Science Institute, said the more than 200 types of STEC are widely distributed in the environment that people share with animals; more than 100 have been linked to human disease.
Validating the effectiveness of commercial antimicrobial technologies to control STEC contamination during meat processing is his area of expertise and the focus of a $25 million U.S. Department of Agriculture grant. Phebus serves on the grant’s management team with collaborators from 15 other institutions. The team recently completed a study on veal processing. Raw veal poses a substantially higher STEC risk than beef, according to the USDA, and Phebus and colleagues have now validated multiple methods of reducing veal risks and beef risks in consumer products.
Phebus’ team collaborates with several processing companies to demonstrate that lab- generated antimicrobial technologies translate into effective real-world tools. Learn more about Phebus’ research.
“Working with industry has made this work really powerful,” Phebus said. “We enhance public health by giving the food industry proven antimicrobial intervention technologies to control STEC and other pathogens on their raw and processed products.”