Study from Kansas State University's Institute of Computational Comparative Medicine challenges traditional food safety assessment method
Tuesday, June 28, 2016
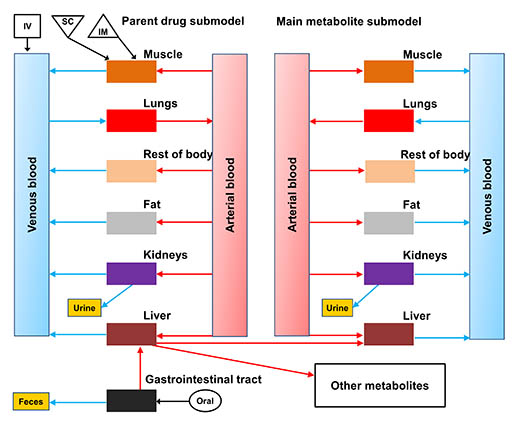
A schematic of a general physiologically based pharmacokinetic — or PBPK — model for the drugs ceftiofur, enrofloxacin, flunixin and sulfamethazine in cattle and swine. | Download this photo.
MANHATTAN —Research by Kansas State University's Institute of Computational Comparative Medicine is challenging conventional thought regarding human food safety and drug residues found in cattle and swine tissues.
In the study "Human Food Safety Implications of Variation in Food Animal Drug Metabolism" published recently in Nature's Scientific Reports, Kansas State University researchers Zhoumeng Lin, Christopher Vahl and Jim Riviere found that diseases can dramatically influence the type of drug residue found in tissues of food animals that are tested by regulatory agencies in monitoring human food safety.
Withdrawal times — the time from last drug administration to slaughter — are determined in healthy animals during the drug development process assuming that the ratio of the marker residue to the total residue produced is constant. This "marker residue ratio" is then used to set legal tissue tolerances in food safety inspection programs, but diseases in treated animals may alter this ratio, said Lin, an assistant professor of anatomy and physiology.
"We created a general physiologically based pharmacokinetic — or PBPK — model for representative drugs such as ceftiofur, enrofloxacin, flunixin and sulfamethazine, which are typically used in cattle and swine and have had residue violations reported," Lin said. "Our simulation results showed that the ratios of the marker residues to the total residues for studied drugs were not fixed values, but time-dependent. Disease changes the ratios substantially, and the degree of change depends on the type of drug, exposure time, tissue and species."
Although it is just one study, the researchers believe their work could have significant impact on food safety in the future, said Riviere, director of the Institute of Computational Comparative Medicine. Riviere also is a university distinguished professor, Kansas Bioscience eminent scholar, the MacDonald chair in the College of Veterinary Medicine, and an elected member of the National Academy of Medicine.
"The cornerstone of regulatory chemical food safety programs is the monitoring of food products for violative chemical residues," Riviere said. "For edible products from food-producing animals, such as meat, milk or eggs, residue concentrations are determined based on jurisdictional-specific regulations that result in the determination of a tolerance or maximum residue level for specific drugs in a specific tissue for specific animal species."
The researchers' model was calibrated for each drug in each species with multiple datasets from the Food Animal Residue Avoidance Databank, which has an office at Kansas State University.
"We based our results on regression analyses between model-simulated and measured plasma and tissue concentrations of parent drugs and/or major metabolites for each drug in each species," Lin said. "In general, disease changed the ratios of ceftiofur, enrofloxacin, flunixin and sulfamethazine by several fold, and the ratio could be different by up to several orders of magnitude at the withdrawal time compared to that at the time right after drug administration."
The results raise a question about the reasonableness of the underlying assumption of using a fixed ratio by the Food and Drug Administration to determine withdrawal times of veterinary drugs in food animals, Riviere said. But he also said the FDA's method is very conservative and safe, and that further study could help provide more accurate data in the future.
Study co-author Vahl is an assistant professor of statistics in the university's College of Arts & Sciences.
The research was supported by the U.S. Department of Agriculture for the Food Animal Residue Avoidance and Depletion Program, USDA 2013-41480-21001, and the Kansas Bioscience Authority. The idea and need for this analysis originated from a review by Riviere on the USDA's Food Safety and Inspection Service's National Residue Program conducted for The Pew Charitable Trusts.
