Dr. Tendai Gadzikwa |
||||
 |
Associate Professor |
|||
| CBC 101 | ||||
| gadzikwa@ksu.edu | ||||
Research Themes  |
||||
| 785-532-6703 (lab) | ||||
Biography |
|
|
2023-Pres. 2016-2023 2015 2012-2015 2009-2011 2003-2009 1999-2003 |
Associate Professor, Kansas State University Assistant Professor, Kansas State University Visiting Researcher, University of Alberta Senior Lecturer, University of Zimbabwe Schlumberger Postdoctoral Fellow, University of Amsterdam Ph.D, Northwestern University B.A. in Chemistry, Macalester College |
Research Overview |
| Supramolecular chemistry, metal-organic framework materials, and catalysis. The members of the Gadzikwa lab consider ourselves “molecular architects” - we use the tools of Inorganic, Organic, and Materials Chemistry to construct functional materials using molecules as building blocks. Like architects, we design the supramolecular structure, then plan and oversee its construction via the self-assembly of molecular building blocks. Finally, we decorate the interior of our construct such that it best fulfils the purpose for which it was built. Our particular focus is supramolecular catalysis. A long-standing goal of the field is the construction of catalysts that more accurately mimic enzyme active sites, i.e catalytic sites that are confined, highly functionalized, and flexible. To this end, our group has been using metal-organic framework (MOF) materials as scaffolds on which we can deliberately organize complex chemical functionality within confined, 3-dimensional space. Our methodology entails the assembly of modifiable organic linkers into sufficiently porous, independently modifiable MOF materials, and their sequential elaboration with multiple additional functionalities.
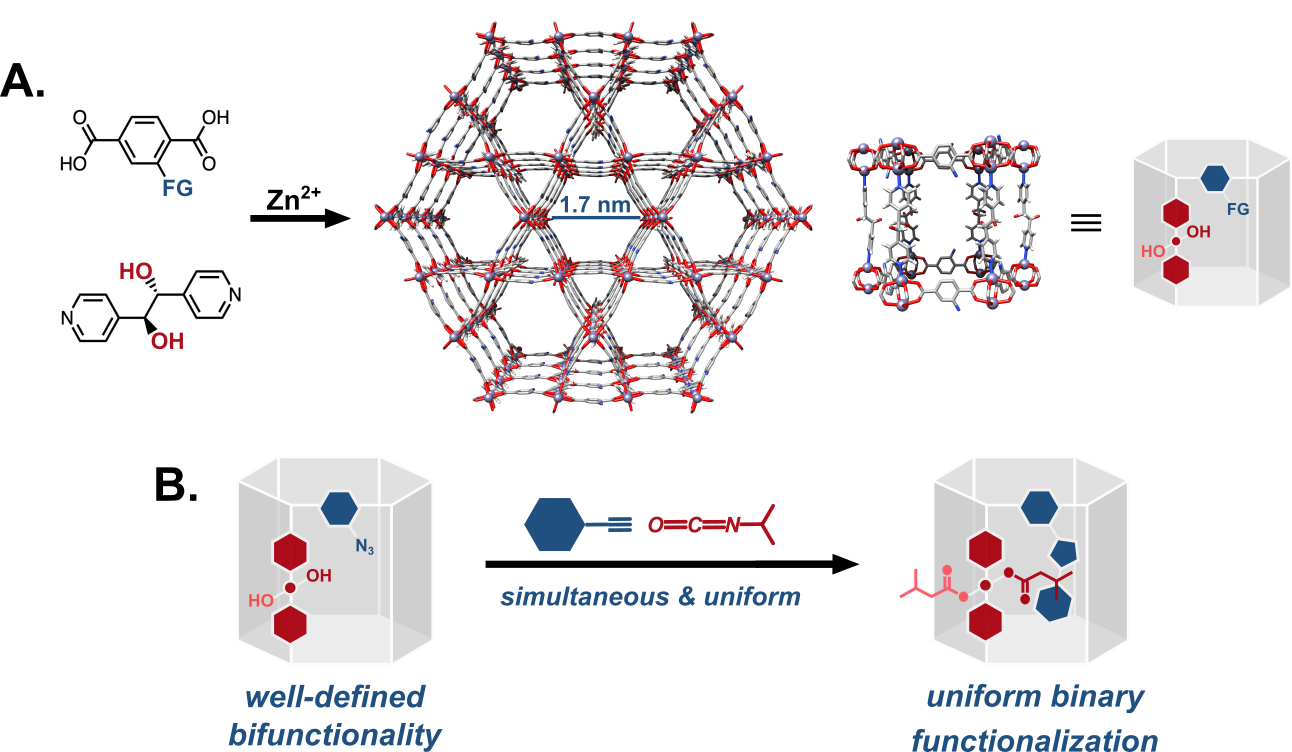 Figure 1. A: The self-assembly of modifiable organic linkers with zinc cations to generate a bifunctionalizable metal-organic framework (MOF) material, KSU-1. B: Schematic representation of the sequential, independent functionalization of KSU-1 to yield a uniformly multifunctional MOF material.
|
Selected publications |
|