The buzz behind the bite: How scientists tackle infectious diseases and the mosquitoes that spread them
Want to be in the middle of a swarm of mosquitoes? Check out an interactive 360-degree video that shows a closer look at research at the Biosecurity Research Institute. (For best viewing experience, use the Chrome or Firefox web browser on a computer or the YouTube app on a mobile device.)
View an interactive 360-degree photo.
By Jennifer Tidball
The numbers are enough to make you feel itchy.
Since 2015, more than 43,000 cases of Zika virus have been reported in U.S. states and territories.
Malaria caused an estimated 212 million clinical cases and 429,000 deaths worldwide in 2015.
West Nile virus has infected an estimated 2.5 million people in North America and caused more than 44,000 U.S. cases since it emerged in 1999.
There’s no question: Mosquitoes may be small insects, but their presence affects the health of billions of people across the world.
“Seventy percent of emerging diseases are zoonotic, and many of them are mosquito- borne,” said Stephen Higgs, director of Kansas State University’s Biosecurity Research Institute. “Mosquito-transmitted viruses are expanding. The viruses that Kansas State University is working on are things that could be next.”
What’s next? The possible list includes names like Japanese encephalitis virus, yellow fever virus or dengue viruses.
No matter the virus and no matter the disease, scientists agree: Advanced research is the solution. From studies of viruses, mosquito biology and insect control, Kansas State University researchers are tackling the unknowns of infectious diseases and the mosquitoes that spread them. They hope to make it easier to manage — and possibly someday eradicate — health threats such as malaria or Zika virus.
“Studying these viruses before they get to the United States gives us the knowledge we need to prepare,” said Dana Vanlandingham, assistant professor of virology. “It’s important to estimate what we think might be coming in, because if it does, we need a plan immediately. Debate and slowness let the diseases establish. Once some of these diseases are here, they’re here.”
Virulence of viruses
| Scott Huang, Dana Vanlandingham and Stephen Higgs study mosquito-transmitted viruses. |
Researcher Scott Huang knows firsthand the effects of infectious diseases. Huang is from Taiwan, where Japanese encephalitis virus and dengue viruses are endemic pathogens.
“Once these viruses are introduced, there is no way to get rid of them because they can be persistent in mosquitoes or susceptible hosts,” said Huang, a university research assistant professor of diagnostic medicine and patho- biology. “These viruses can infect humans and animals without showing symptoms, which makes them some of the hardest targets to control.”
That’s part of what motivates Huang to study mosquito-transmitted viruses at the university’s Biosecurity Research Institute, a biosafety level-3 facility where scientists can safely study animal and human infectious diseases. Higgs, Vanlandingham and Huang collaboratively are studying Zika virus, Japanese encephalitis virus, yellow fever virus and Cache Valley virus with researchers from institutions in the U.S. and the U.K.
The university scientists were part of a multi-institutional team that recently developed a possible new Zika virus vaccine and published the results in Nature. The immunogenic vaccine potentially could protect against the virus with one dose and could become a tool to prevent future outbreaks, Higgs said.
The Biosecurity Research Institute team played an important role during the Zika virus public health emergency in 2016. The institute has facilities needed to study mosquitoes and understand how they become infected with Zika virus.
Through a research project with Ross University, along with funding from the National Institutes of Health, the scientists are providing skills and expertise for further studies with Zika virus and chikungunya virus.
But the university work extends to other emerging viruses as well.
Higgs, Vanlandingham and Huang are performing several studies — funded by the National Bio and Agro-defense Facility Transition Fund and the Swine Health Information Center — on an emerging type of Japanese encephalitis virus. Their studies are the first U.S. studies of Japanese encephalitis since the 1940s and the researchers also are doing the first studies with the Cache Valley virus that is present in North America.
Japanese encephalitis virus is found primarily in pigs and birds in Asia, but it can transmit to humans and cause severe inflammation, or encephalitis, of the brain.
The Japanese encephalitis virus research at the Biosecurity Research Institute is especially important: A new, emerging type killed 14 people in China in 2014. Other older strains of Japanese encephalitis virus constantly are circulating in Asia and infect an estimated 67,900 people per year. Although many people in Asia are vaccinated, the vaccine is not very effective because it is made for the older strains, researchers said.
“As the virus continues to circulate, the threat still remains,” Higgs said. “The new strain is just as bad as the old strain. If a strain is going to be introduced to the U.S., it’s going to be this new strain and the U.S. is not prepared for a potential outbreak.”
Through U.S. Department of Agriculture funding and collaboration with the Arthropod-Borne Animal Diseases Research Unit in Manhattan, the researchers are determining if North American mosquitoes could transmit Japanese encephalitis virus and how the U.S. could prevent an outbreak.
The team has published results in Vector-Borne and Zoonotic Diseases and PLOS Neglected Tropical Diseases. The Japanese encephalitis virus work is a transition project that will jump-start research at the National Bio and Agro-defense Facility, or NBAF, the U.S. Department of Homeland Security’s foremost animal disease research facility that is being built adjacent to the university’s Manhattan campus.
Higgs, Vanlandingham and Huang’s research on Cache Valley virus aims to increase limited knowledge of the virus’s transmission cycles. The virus is an important agriculture pathogen that primarily affects sheep and is widespread in North America.
With no approved vaccine or treatment, Cache Valley virus’s biggest human public health concern is its potential to cause neurotropic diseases, which can lead to permanent nerve damage, Huang said. The Biosecurity Research Institute work aims to identify potential carriers that could transmit Cache Valley virus. The USDA- sponsored project is a collaborative effort with U.K. researchers.
Back to the biological basics

| Bart Bryant and lead researcher Kristin Michel are targeting the mosquito immune system in their research. |
One way to fight mosquito-borne diseases like malaria, yellow fever or dengue fever is to make the insect its own worst enemy. University biologist Kristin Michel has a specific target in mind: the mosquito immune system.
Michel, associate professor of biology, and Bart Bryant, research assistant professor of biology, are studying Anopheles gambiae, the mosquito species that is the main transmitter of malaria
in sub-Saharan Africa. Their goal is to identify ways to eliminate pathogens and parasites in the mosquito before it can transmit them to humans.
Michel and Bryant approach their work like a puzzle: Figure out what molecules are in the immune system, how they function and what immune responses they control. Then determine how these pieces fit together to contribute to the mosquitoes’ immunity as a whole and how they relate to the pathogen.
Their NIH- and USDA-funded research has determined the key role of proteases and their inhibitors in controlling humoral immunity as well as the role of hemocytes in cellular immunity.
Michel’s team recently showed that in the first 24 hours after a mosquito eats, the hemocytes — or blood cells — increase in a mosquito’s immune system as it prepares to fight any pathogens from the blood.
“Imagine if every single time you eat a meal, all your white blood cells double,” Michel said. “It would have to be a massive infection or inflammation response, and mosquitoes do that every time they take a blood meal.”
Bryant now is using NIH funding to develop a gene therapy-type approach to turn off specific genes in specific tissues in Anopheles gambiae.
“We want to better understand what a gene is doing and what its role is in one tissue versus another,” Bryant said. “We’re trying to come up with a unique way to better regulate the expression of a gene.”
A better genetic understanding could help stop the spread of malaria, yellow fever and dengue fever. Scientists could maximize mosquito control strategies while minimizing side effects for the insect.
“Malaria is a global problem,” Michel said. “People agree that the existing control methods will not lead to elimination of malaria. We need to accelerate our efforts and for that, we need to continue to do research. Our team wants to contribute — even if it’s a tiny bit — to that mission.”
Going with the gut
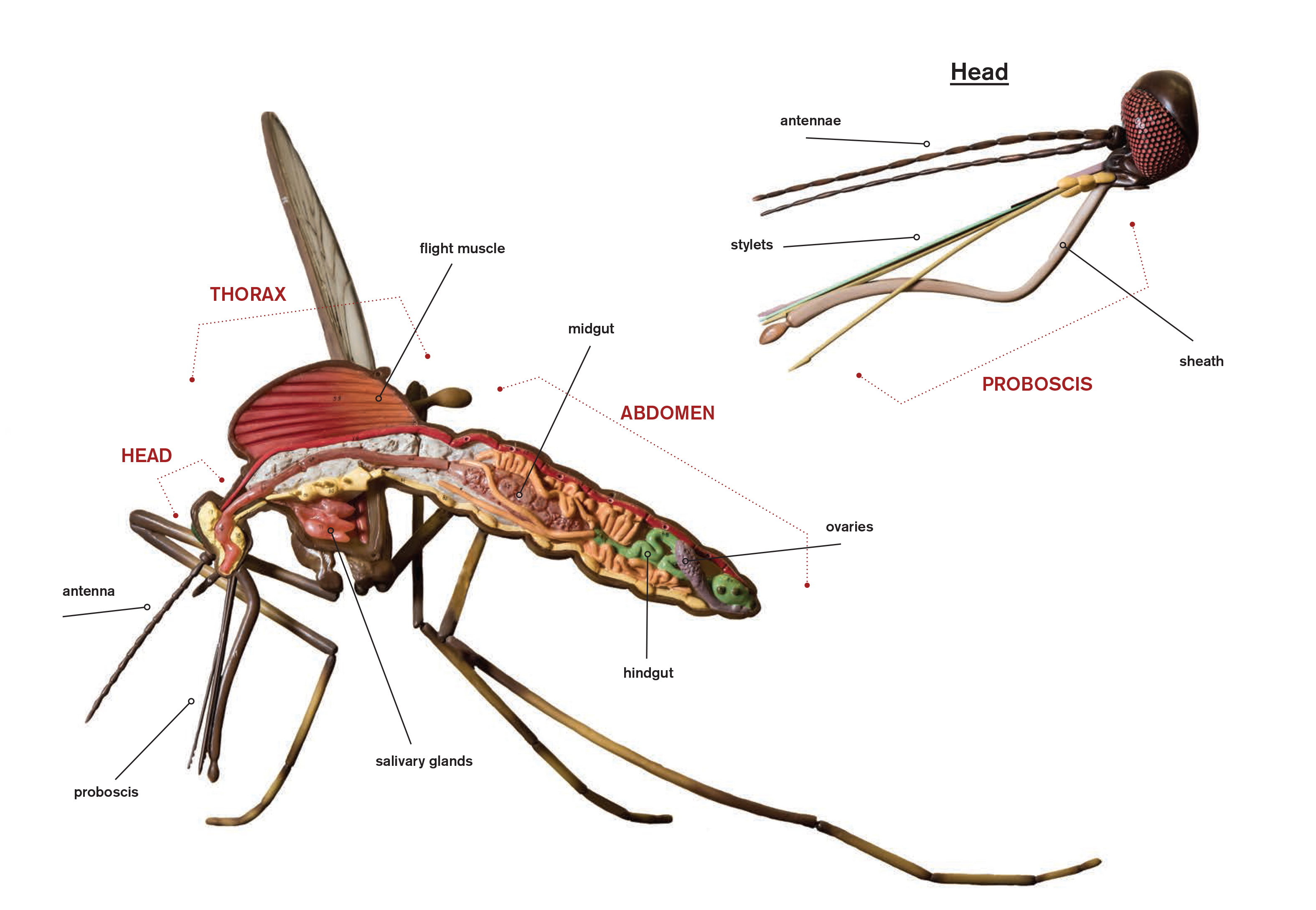
| The anatomy of a mosquito View a larger image. |
Only a few dozen of the more than 3,000 species of mosquitoes in the world can transmit viruses. To understand why, two biology professors — Rollie Clem and Lorena Passarelli — are investigating the insects’ intestines.
It turns out that the gut and the surrounding structure — called the basal lamina — are a mosquito’s key defense against viruses. Through NIH funding, Clem and Passarelli have studied the basal lamina and its network of proteins to understand how it can act as a barrier against viruses and how that barrier is disrupted during a blood meal.
When a mosquito bites a host, any viruses in the blood travel through the mosquito digestive system to the gut. Sometimes viruses are able to escape the gut, infect the salivary glands and shed in the saliva when the mosquito bites another host. Yet other times, viruses are not able to leave the gut and the mosquito does not spread the virus. The biologists want to know how the virus can escape the gut and travel into the main body.
“We previously identified enzymes that were necessary for midgut escape in an insect virus,” Passarelli said. “We are now investigating whether mosquito-vectored viruses use the same enzymes to facilitate midgut escape.”
Specifically, the researchers are studying Sindbis virus, a mosquito- transmitted virus that can cause mild symptoms in humans, such as fever or a rash. The biologists use an arthropod containment level-2 facility to safely study Aedes aegypti mosquitoes.
By looking at enzymes involved in allowing midgut escape by Sindbis virus, the researchers could apply any gained knowledge to other Aedes aegypti-transmitted diseases, including Zika virus, dengue fever and yellow fever.
“Many of these diseases are in other countries, but certainly are threatening to come into the U.S.,” Clem said. “With changing climates, these mosquitoes are expanding their range farther and farther north. There is a lot of public awareness about the importance of these diseases and our research could help the millions of people affected by these viruses every year.”
Investigating insect control

| Aedes aegypti mosquito |
Of course, a key way to keep mosquito-borne diseases under control is to control the insects that spread them.
That’s where Kun Yan Zhu, professor of entomology, fits in. His work starts with chitin, a major chemical component of a mosquito’s exoskeleton shield. When insects are not able to produce chitin, they can’t survive. But when insects produce a reduced amount of chitin, they may become more susceptible to insecticides.
“The exoskeleton is the first defense line for mosquitoes and other insects,” Zhu said. “Chitin biosynthesis is an important target for insect control. We are trying to understand chitin biosynthetic pathways and develop new techniques to prevent chitin production using chemical and genetic approaches.”
Zhu and his team recently patented a form of nanoparticle insect control that uses a genetic chain reaction to prevent mosquitoes and insects from producing chitin.
“Insect control that targets chitin biosynthesis is safer and less likely to affect humans because we do not produce chitin,” Zhu said.
Zhu’s research also is attacking another major problem in mosquito control: insect resistance to insecticides. Over time, mosquitoes can become resistant to insecticides, which have to be replaced with newer versions.
Using USDA and other funding, his team is investigating a family of enzymes that helps mosquitoes detoxify chemicals. The scientists are trying to find possible connections between the insecticide structure and the detoxification enzymes in Aedes aegypti mosquitoes to better understand how the mosquitoes become insecticide-resistant.
“By understanding detoxification mechanisms, we will be able to selectively use insecticides that may be able to control resistant insects, including mosquitoes,” Zhu said. “This is an important strategy to control some of the most devastating disease-spreading insects in the world.”
Read the rest of Seek and see the PDF version of this story from New Prairie Press.
Want to be in the middle of a swarm of mosquitoes? Check out this interactive 360-degree photo that shows a closer look at research at the Biosecurity Research Institute.
Post from RICOH THETA. - Spherical Image - RICOH THETA